Gene drives – technologies for spreading genetic modifications throughout populations
- Project team:
- Thematic area:
- Topic initiative:
Committee on Education, Research and Technology Assessment
- Analytical approach:
TA project
- Startdate:
2020
- Enddate:
2024
The final report on the TA project was approved by the Committee on Education, Research and Technology Assessment on 4 December 2024.
Gene drives – technologies for spreading genetic modifications throughout populations

At a glance
- Gene drives have the potential to help solve major challenges, including the fight against invasive species or vector-borne diseases such as malaria.
- These hopes are offset by technical and scientific challenges as well as environmental risks.
- For countries or regions wishing to take the step towards the necessary field trials, defining acceptable conditions for the approval and implementation of such trials is likely to be the greatest challenge, given the controversial and polarised public and scientific debate.
- Options for action include further research into controllable gene drive systems and the development of methods and guidelines for assessing efficacy and potential environmental risks.
sprungmarken_marker_4719
What is involved
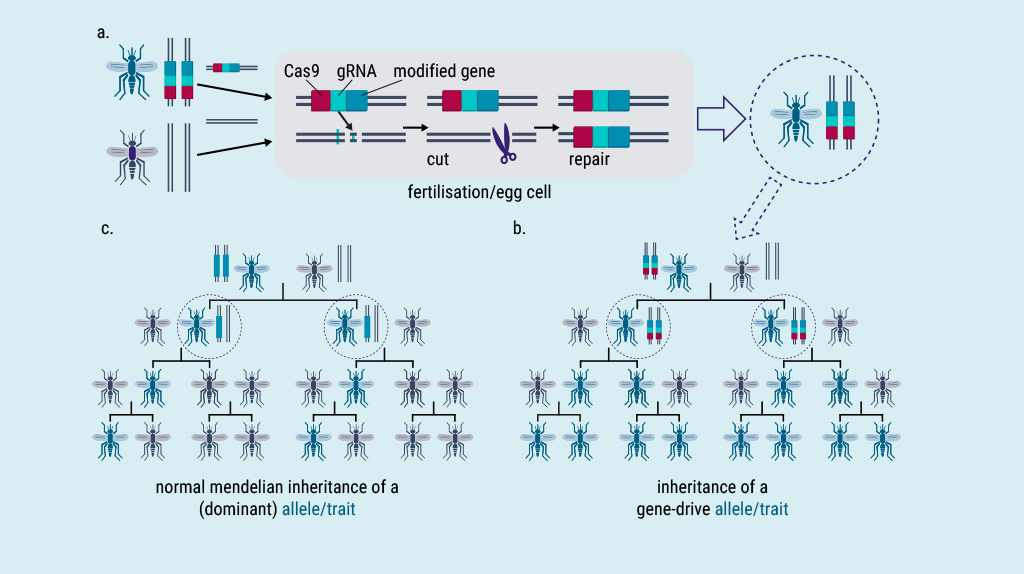
The term gene drive describes a phenomenon in which certain segments of genetic material (genome) are passed on at an overly high rate. These genome segments and the traits they determine can spread through populations of organisms within a few generations (Fig. 1).
Gene drives can be based on various naturally occurring or genetically engineered (synthetic) DNA segments and associated genetic mechanisms. Synthetic gene drive systems are being developed in a range of organisms for different objectives or purposes. One possibility is to greatly reduce or eliminate populations of individual species through so-called suppression gene drives, in particular of malaria-carrying mosquitoes for disease control, of invasive rodent species for nature conservation, and for the control of insect pests in agriculture. Research is also underway to genetically modify populations of disease-carrying mosquitoes or weeds using modification gene drive systems so that they no longer transmit diseases efficiently or become sensitive (again) to pesticides.
However, there are a number of challenges that make it difficult both to develop possible gene drive applications and to assess their potential and risks. Questions about possible benefits and risks, such as the spread of gene drives beyond the target population and the associated damage to ecosystems, make gene drive applications controversial. With the advent of CRISPR/Cas-based genome editing techniques, which have greatly facilitated the targeted genetic modification of various organisms to produce different gene drive systems (Fig. 2), research into the development of such systems for various applications, and the controversial scientific and public debate about them, have intensified considerably over the last 10 years or so. In addition to civilian applications, the possibility of malicious terrorist or military use of gene drives is often discussed, raising the question of the dual use of gene drive research.
Abb. 1 Typical functioning of a synthetic gene drive system
Possible gene drive applications: the potential for solving complex problems
Discussions on gene drive applications tend to focus on invasive species and malaria, where new or additional control measures are considered particularly necessary and where plausible application scenarios for gene drives and their potential benefits are emerging. With regard to gene drive systems for use in agriculture, it has not yet been possible to develop systems for relevant insect pests that work efficiently in (laboratory) populations, and synthetic gene drive systems in agriculturally relevant plants, i.e. weeds, have only been described theoretically to date. Given this early stage of development, it is not yet possible to analyse the potential benefits and risks for this area of application any further..
Combating invasive species
With regard to the problem of invasive rodent species for ecosystems, especially on islands, gene drives could be an attractive option, especially as previous approaches to solving the problem have been fraught with difficulties. To date, invasive rodents have primarily been controlled through the large-scale use of poisoned baits. This approach is expensive, not species-specific and ethically controversial. Particularly on inhabited islands, the organisational and financial effort required to ensure the best possible protection of people, pets, livestock and other non-target species (including wild birds) is enormous. In addition, the painful poisoning of rodents does not initially meet with the approval of many local residents, so a complex process is needed to address concerns in order that the measures are supported by all affected, and the resulting rules of conduct and restrictions are supported. By contrast, gene drives are unlikely to pose a threat to other animal species or humans and could solve the animal ethics problem, as they do not involve the painful killing of animals, but simply prevent further reproduction.
Fighting malaria
Current strategies to combat human malaria are also far from adequate or have stalled, both in terms of potential global eradication and local/ regional control or elimination in the regions most affected by the disease. This is particularly true for countries in sub-Saharan Africa, which still account for around 95 % of global malaria infections and more than 600,000 deaths per year (80 % of which are in children under five). All previous strategies, including the mainstays of insecticide-based vector control and drugbased infection prevention, have failed to bring the disease under control in Africa’s high-endemic areas. The use of the only partially effective childhood vaccines is unlikely to achieve this either. Experts agree that new, additional strategies and measures are needed, with the ultimate goal of eradicating the disease. Key institutions in the fight against malaria, such as the World Health Organisation (WHO), consider gene drives to be a promising additional option in the the fight against malaria that deserves support. As a result, the Target Malaria Consortium (a research network that aims to use gene drives to control malaria-transmitting mosquito species in sub-Saharan Africa) has received a recommendation from the WHO to continue its gene drive developments and to specify further testing steps.
Challenges in developing gene drive applications
The positive expectations expressed by various stakeholders regarding the future potential of gene drives are offset by a number of challenges that make the actual contribution to solving problems at least uncertain. Scientific and technical difficulties and safety issues The main challenge in controlling invasive rodent species on islands (mice, rats) is that although the first gene drive systems for mice have been developed, they have either been less effective than hoped for in the laboratory or (according to model calculations based on laboratory tests) would probably take a relatively long time to eliminate populations. In addition, the far greater damage caused by invasive rodents on islands is caused by rats and not by mice. However, little is known about the genetic diversity and the natural behaviour of invasive rat species, such as the house rat, in the newly colonised habitats (e. g. in Australia). Thus the chances of success in developing a gene drive system for rats are (still) less certain than for mice. In addition, a major challenge is to secure such gene drive systems so that they do not lead to the eradication of local mouse or rat populations on the mainland or other continents, if gene drive individuals escape from the target island or are deliberately (illegally) moved to other locations.
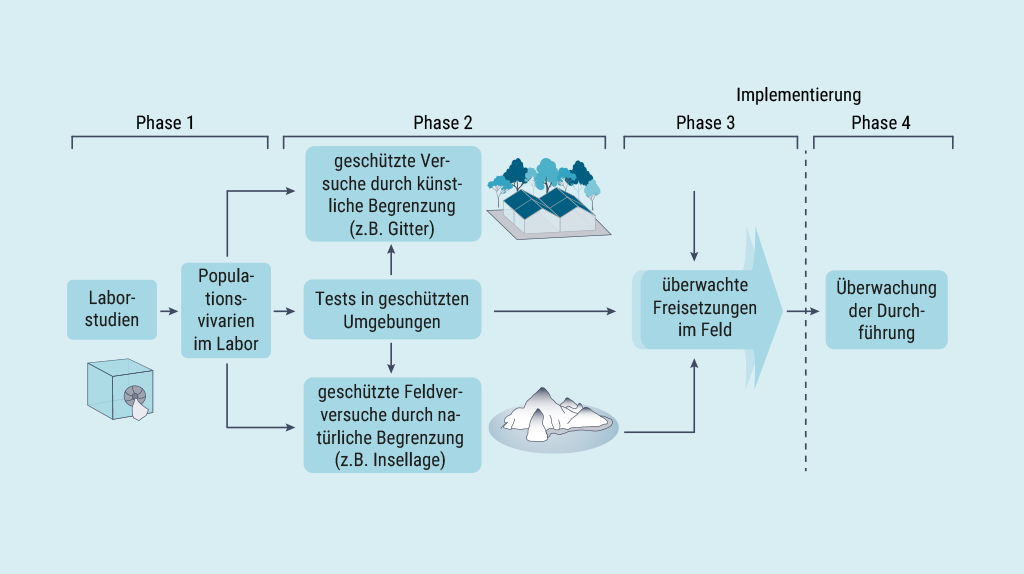
Both for applications against invasive rodent species on islands and for population suppression or modification of relevant malaria-transmitting mosquito species, for which efficient gene drives have already been developed and successfully tested with populations in the laboratory, field experiments are needed for further development. Without these, it is difficult to assess the real potential of these approaches in the intended areas of application. This applies both to assessing the efficacy and the possible unintended ecological consequences of eliminating endemic mosquito populations or gene drive mice that could escape from islands. Computational models can contribute to some extent to the assessment of the safety of gene drive systems and the environmental risks, but they cannot be validated or substantially improved without data from field trials.
The dual-use potential of gene drives – the possibility that they could be used for malicious terrorist or military purposes – is a recurring theme in the debate. Given the very early stage of development of the systems, it seems questionable whether they could actually be directly misapplied in the near future to pose a serious threat with far-reaching potential consequences, e. g. for public health or national security, and therefore need to be classified as „dual-use research of concern“. Overall, gene drive systems are unlikely to be very attractive for both terrorist and military purposes, mainly because of the complex development process that needs to be adapted to each target species and its ecological characteristics, the sometimes very long timescales for effects to unfold in populations, and the uncertainties regarding the expected outcomes.
Regulatory and political challenges
For countries or regions wishing to take the step of conducting initial field trials of synthetic gene drive systems – which in principle fall under the existing regulations for genetically modified organisms (GMOs) – the biggest challenge is likely to be defining or agreeing on the conditions for the authorisation and implementation of such trials. Whether a country wishing to use and release gene drive organisms would need to obtain the consent of neighbouring countries (which may also be affected by the release) is at least currently uncertain. The Cartagena Protocol on Biosafety (a follow-up agreement to the United Nations Convention on Biological Diversity), which is legally binding to the Parties to the Protocol, stipulates that the intentional transboundary movement (i. e. the import or export) of biotechnologically modified organisms for release requires the prior consent of the receiving state(s). However, it is not yet clear whether the transboundary spread of gene drives can also be interpreted in this way.
In view of the controversial and polarised public debate, it is to be expected that specific projects for the application of gene drives will meet with clear rejection in some cases. Both proponents and critics agree that science has a central role to play in assessing this new technology and its possible risks, and despite some differences, all actors see the need for international regulation and the organisation of participatory processes. In essence, however, there are two opposing interpretations: gene drives as a promising and useful tool versus gene drives as a danger, particularly due to the potential uncontrollability of (at least some) gene drive systems and the associated potential environmental damage. This naturally leads to different attitudes to possible governance measures (e. g. with regard to a moratorium) or to the question of whether field trials can be conducted safely at all.
Fig. 2 Phase plan for testing and implementing gene drives against malaria
Options for action
Despite the different stages of the development in the possible fields of application and their inherent differences, some overarching policy options for dealing with the entire field of gene drives can be derived. These focus on the cross-application problem of researching and assessing or evaluating the expected efficacy, the possible ecological effects that could transcend national borders, and thus the potential benefits and risks overall.
At the international level, more concrete options for action could include:
- (Further) support the development of guidelines for risk assessment of GMOs with gene drives under the Convention on Biological Diversity, which could complement existing regulations in the most specific and differentiated way possible. In particular, it could be useful for the guidelines to distinguish appropriately between the various possible applications (e. g. elimination of invasive versus endemic insect pests) and gene drive systems (potentially non-confinable versus confinable systems).
- The promotion of a global gene drive (project) registry, as proposed by various experts and organisations, to support and improve the coordination of research, the monitoring of environmental impacts and the democratisation of access to information.
At European and national level, options could aim to better prepare for the challenges posed by climate change through the increase in vector-borne diseases (e. g. caused by the dengue virus) and invasive pest species in agriculture (e. g. Mediterranean fruit fly and spotted wing drosophila). In this context, the following options could be considered:
- Funding for research into various new methods that can diversify the existing portfolio of approaches to mosquito control (in particular the use of chemical pesticides or the Bti method, which is based on an active ingredient from the bacterium Bacillus thuringiensis and that is widely used in Germany). Such complementary approaches could be the subject of a specific funding programme for genetic biocontrol approaches, including gene drive approaches. Research into confinable gene drive systems could be a funding priority for gene drive research.
- The initiation of a multi-stakeholder process as a basis for the most differentiated and concrete problem formulation for the environmental risk assessment (ERA) of gene drive-based systems. In view of the polarised debate on gene drives, a problem formulation implementation of risk analyses and for the evaluation and assessment of ERA data would be an important prerequisite for reducing the risk of ongoing conflicts such as those known from green biotechnology.
Downloads
|
TAB-Fokus no. 48 Gene drives. Technologies for spreading genetic modifications throughout populations(PDF) The policy brief TAB-Fokus offers a compact overview of the content and results of our TA analyses on four pages.
|
|
|
TAB-Arbeitsbericht Nr. 214
The report provides a comprehensive and well-founded information base on the current state of development, potential applications, and safety and regulatory issues of gene drive systems. Options for action are derived from the challenges posed by the development of such systems and from the assessment of possible benefits and risks, in particular with regard to research funding and a risk assessment process that is as widely accepted as possible.
|
25 April 2022
17.00 Uhr - 18.30


